Ad26 vector-based COVID-19 vaccine encoding a prefusion-stabilized SARS-CoV-2 Spike immunogen induces potent humoral and cellular immune responses | npj Vaccines
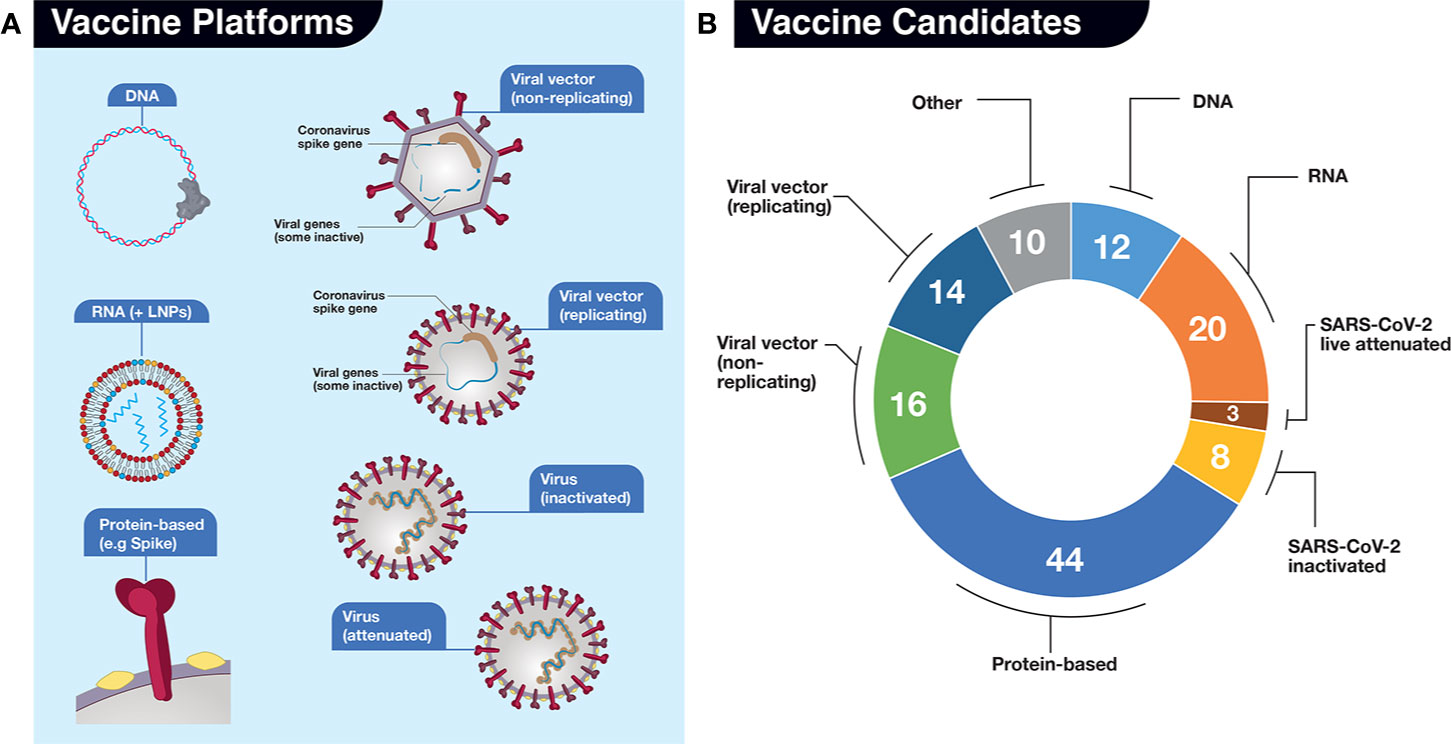
Frontiers | A Snapshot of the Global Race for Vaccines Targeting SARS-CoV-2 and the COVID-19 Pandemic
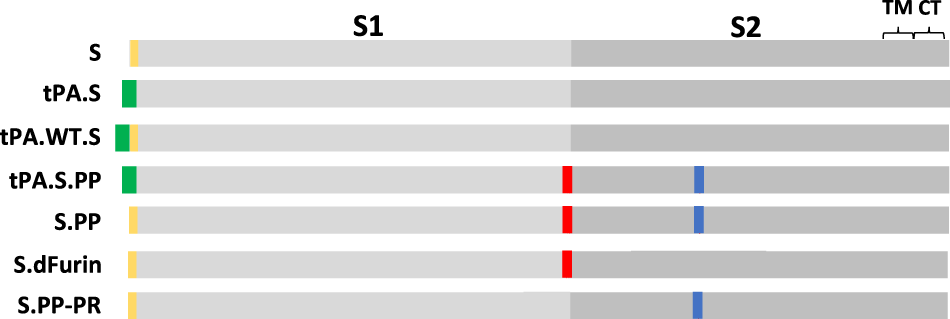
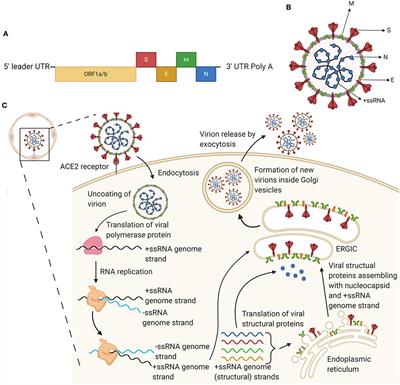
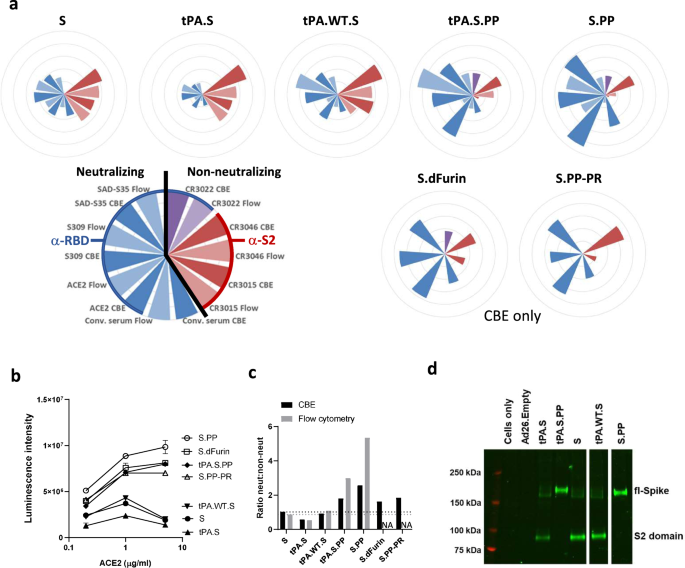
Ad26 vector-based COVID-19 vaccine encoding a prefusion-stabilized SARS-CoV-2 Spike immunogen induces potent humoral and cellular immune responses | npj Vaccines
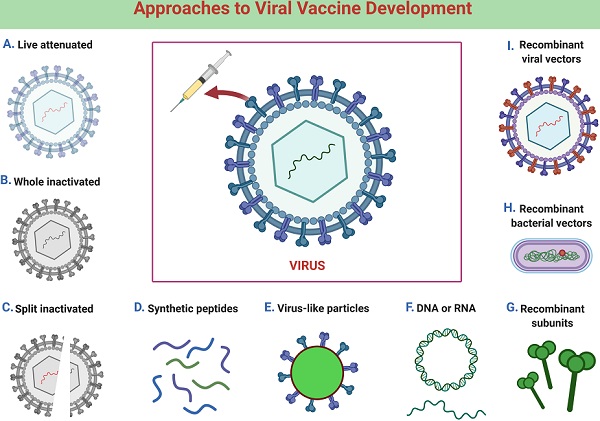
Vaccines | Free Full-Text | COVID-19 Vaccines (Revisited) and Oral-Mucosal Vector System as a Potential Vaccine Platform
AdventHealth begins recruiting participants for Phase 3 COVID-19 Investigational vaccine clinical trial | AdventHealth Research Institute
Vaccines and Related Biological Products Advisory Committee February 26, 2021 Meeting Briefing Document- Sponsor
P00081 Modification to MCDC OTA No. W150KN-16-9-1002 BETWEEN JANSSEN PHARMACEUTICALS, INC. 1125 TRENTON-HARBOURTON ROAD TITUSVIL
Latest COVID-19 vaccine based on technology tested for safety & immunogenicity in IMI Ebola projects | IMI Innovative Medicines Initiative

Johnson & Johnson 'Pauses' COVID-19 Vaccine Trial Due To Unexplained Illness In Participant; Eli Lilly Reportedly Suspending Trial Of Antibody Treatment - Health Policy Watch