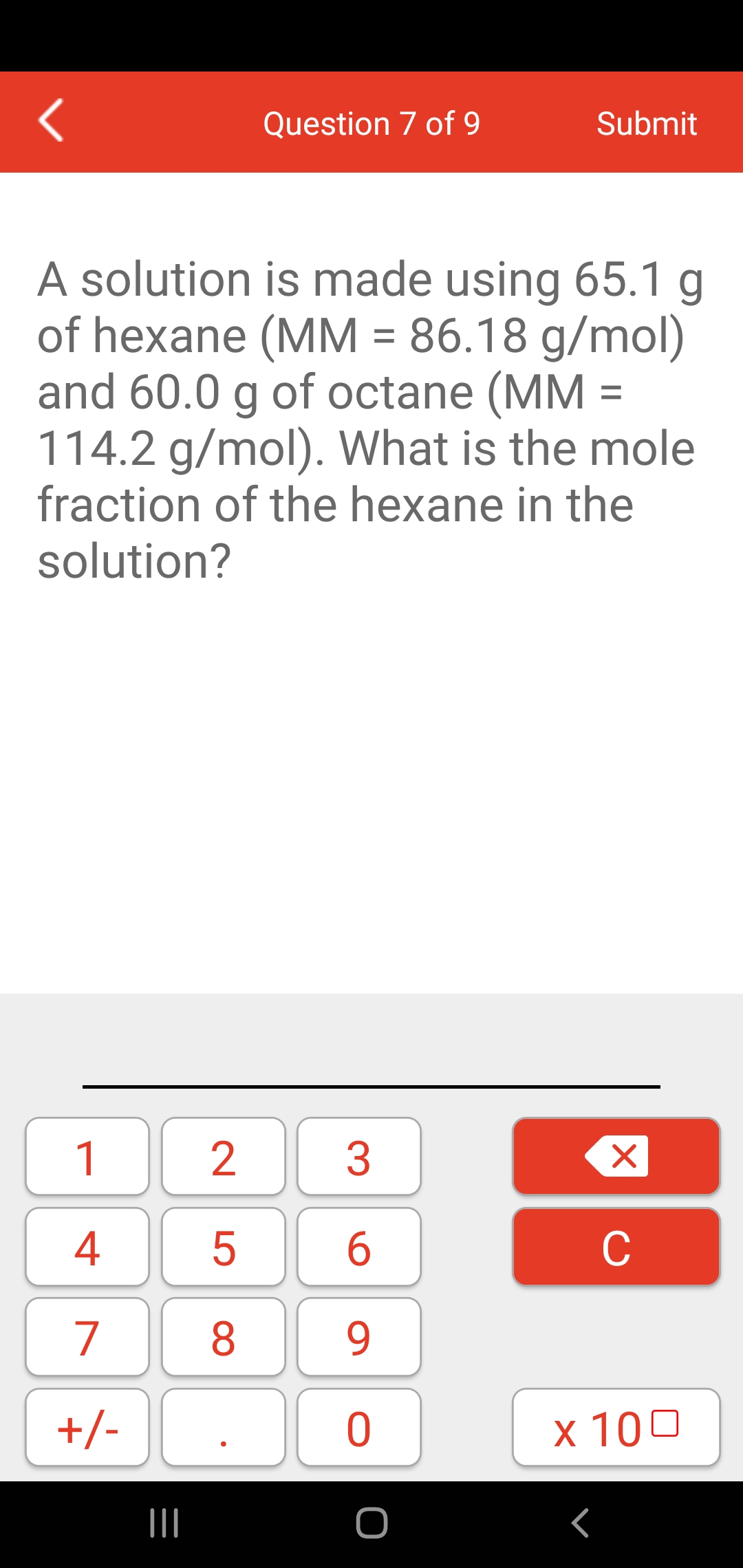
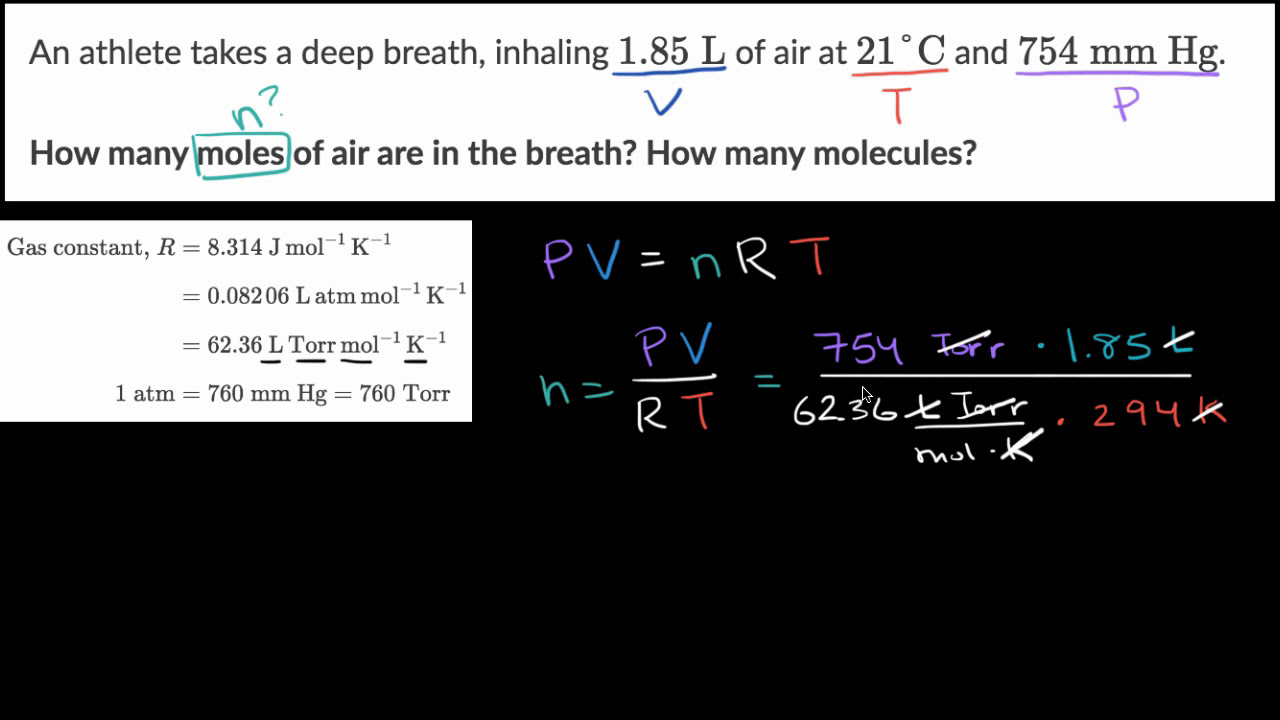
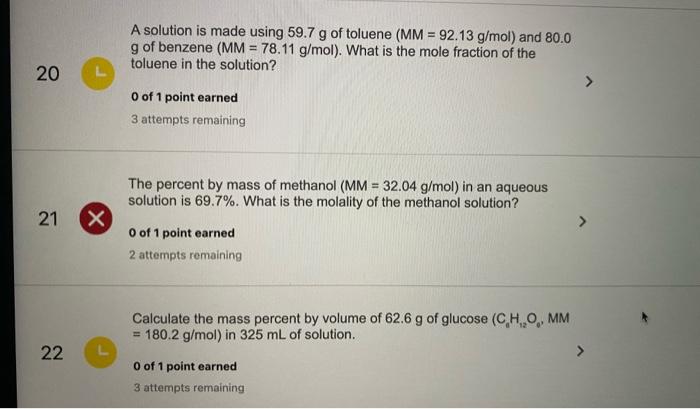
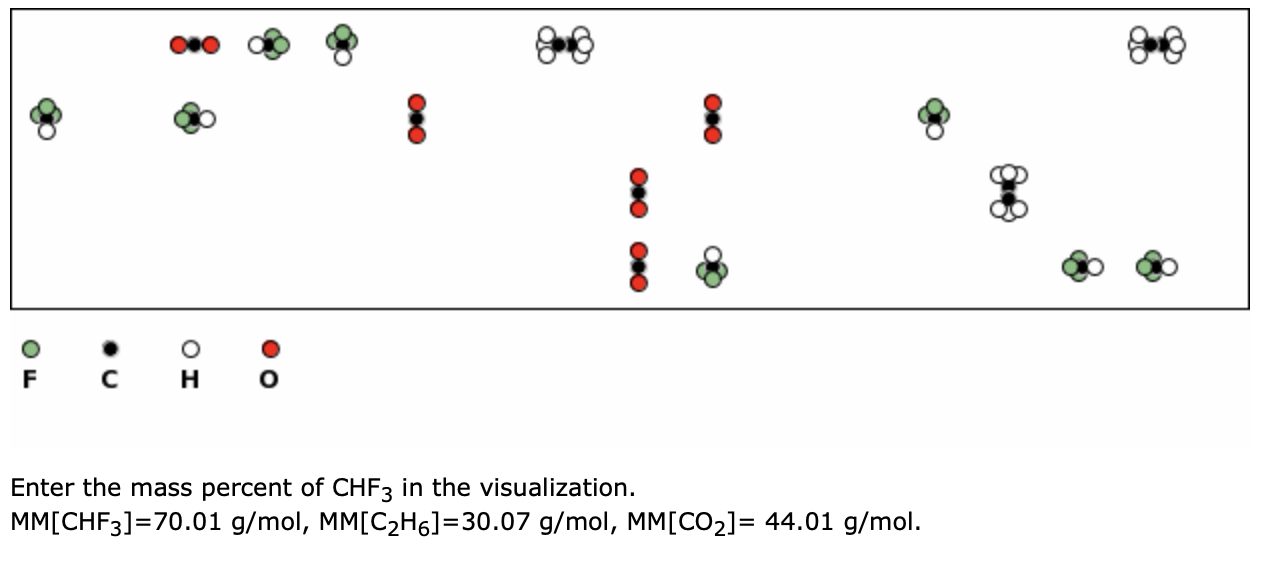
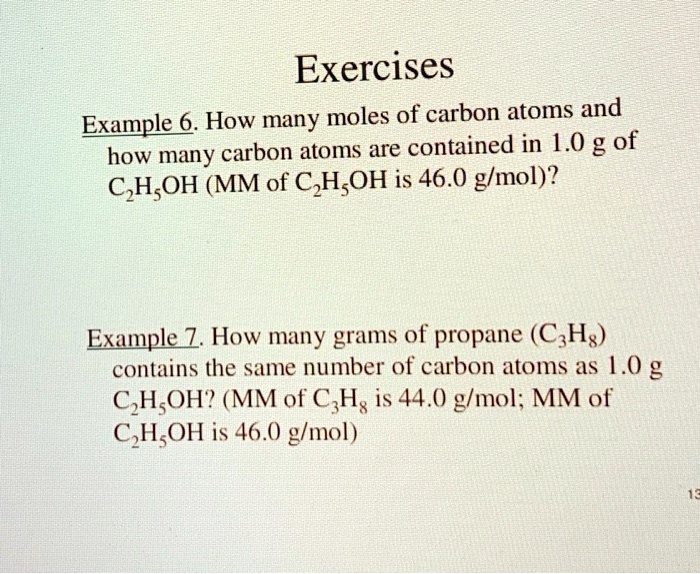PLEASE HELP QUICKLY!!! MM H2O2 = 34.02 g/mol MM H2O = 18.02 g/mol MM O2 = 32 g/mol 2H2O2 —> 2H2O + - Brainly.com

Bahco Wrench 9029C BH9029C Adjustable MOL Central Gran AP Wrench, Silver/Black, 6 Inch, 32 mm - - Amazon.com

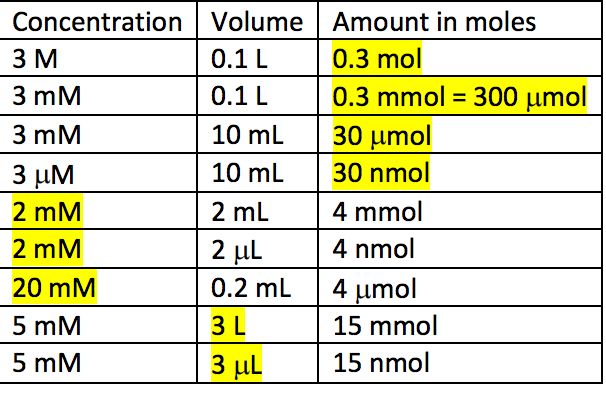
Chapter 5 A Matter of Concentration. Ionic Phenomena = Things that happen to ions, which can be observed. - ppt download

Mole Practice 1. How many particles of gold are in 2.3 moles of Au? 2. Calculate the number of moles - Brainly.com

SOLVED: If 50.0 g of CH₃OH (MM = 32.04 g/mol) are added to a 500.0 mL volumetric flask, and water is added to fill the flask, what is the concentration of CH₃OH